Duke University Medical Center
At the Forefront of Lung Cancer Detection
Duke University Medical Center was one of the first facilities in the country to institute a Lung Cancer Screening Program. As a leader in thoracic imaging, Duke is looked to as a thought leader and reference site for institutions initiating similar programs. As an American College of Radiology (ACR) designated center for Lung Cancer Screening, Duke’s screening program is recognized for providing safe, effective care for at-risk lung cancer patients, While maintaining the highest possible standards.
The shift toward the implementation of lung cancer screening programs began in 2011 with the release of the National Lung Cancer Screening Trial (NLST) results. The study concluded that annual screening with low-dose computed tomography (CT) could detect lung cancer in its earliest stages, reducing lung cancer deaths by 20 percent.
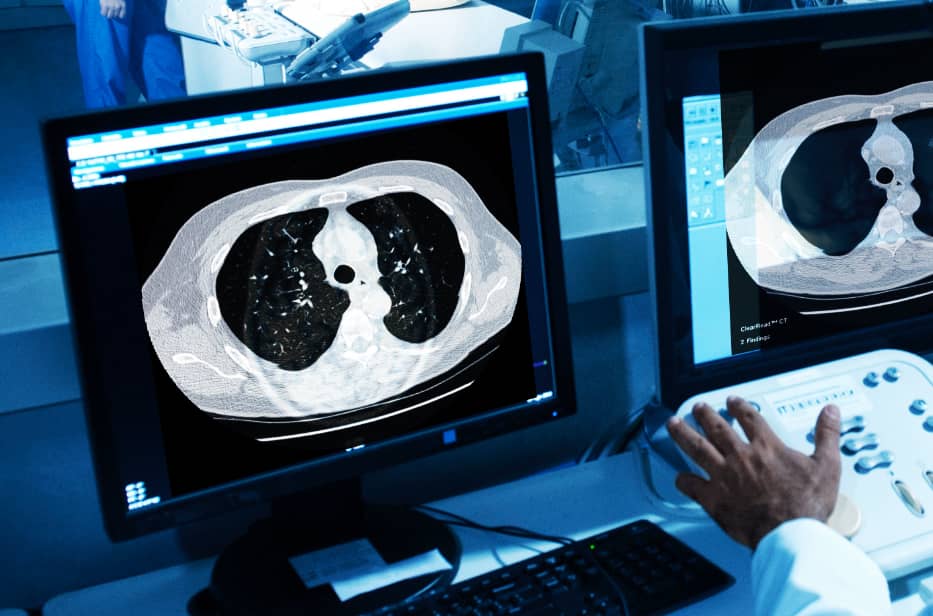
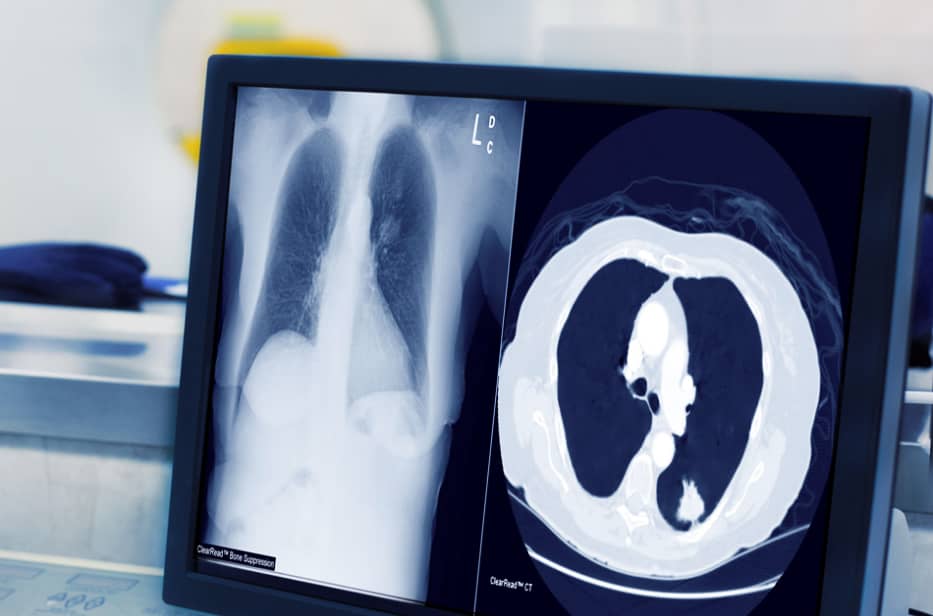
Duke prides itself on providing its patients with the highest quality of care through state-of-the-art technology, programs and the implementation of ground-breaking technologies such as Riverain Technologies’ ClearRead CT, an aid for detecting lung nodules more efficiently and accurately.
Extending Early Detection Beyond Lung Cancer Screening
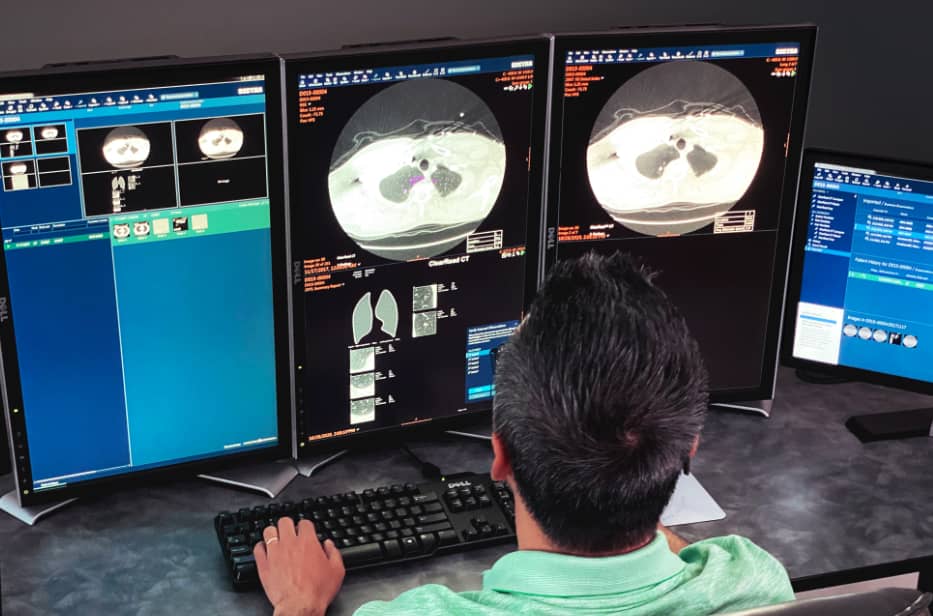
According to Jared Christensen, MD, MBA, Division Chief of Cardiothoracic Imaging and Director of Duke’s Lung Cancer Screening Program, one of their biggest challenges in thoracic imaging is searching for lung nodules. For every chest CT exam, radiologists must search for lung nodules. The detection of incidental pulmonary nodules serves as a critically important opportunity for early lung cancer detection. In an eff ort to help their radiologists detect more nodules more efficiently, Duke has deployed Riverain’s ClearRead CT software throughout their entire health network across all chest CTs, whether taken for screening or not.
Improved Detection, Decreased Reading Time, Standardized Patient Care
ClearRead CT is the only FDA-cleared device to support concurrent reading, allowing for faster reading with clinically proven superior automatic nodule detection performance for all primary nodule types. Detected nodules include: solid, subsolid and ground glass. with the use of ClearRead CT, organizations like Duke are able to achieve a 26 percent reduction in reading time while improving nodule detection by 29 percent. Duke has deployed Riverain’s ClearRead CT software throughout their entire healthcare network, providing a standard of care that supports earlier detection of lung cancer across their entire patient population. ClearRead technology seamlessly processes CT studies from all 15 of Duke’s CT scanners independent of manufacturer or acquisition protocols.