Our technology is revolutionizing radiology. We’d love to work with you to help create better outcomes for patients everywhere.
The first FDA-cleared device that supports concurrent reading, allowing for faster reading with proven, superior, automatic nodule detection.
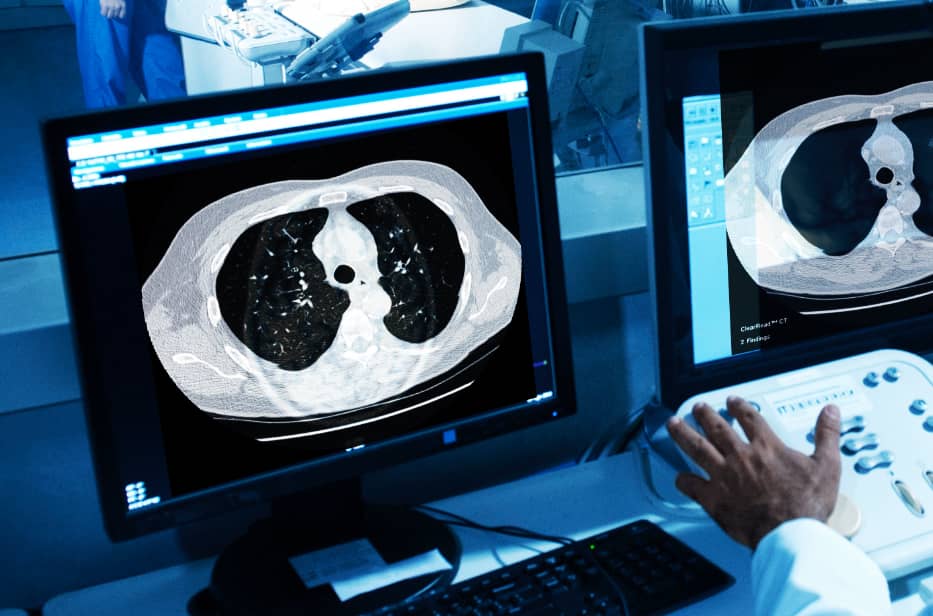
Our five FDA-cleared applications are designed to improve reading efficiency and accuracy across the hospital enterprise without additional equipment, procedures or radiation dose.
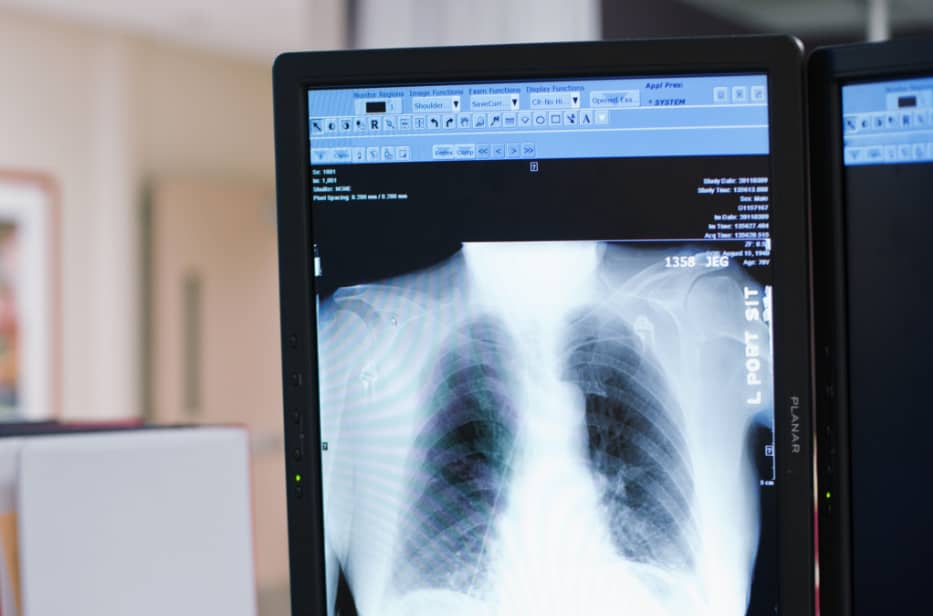
Riverain’s deep learning AI technology is FDA cleared, field tested, and clinically proven to provide real results in radiology.
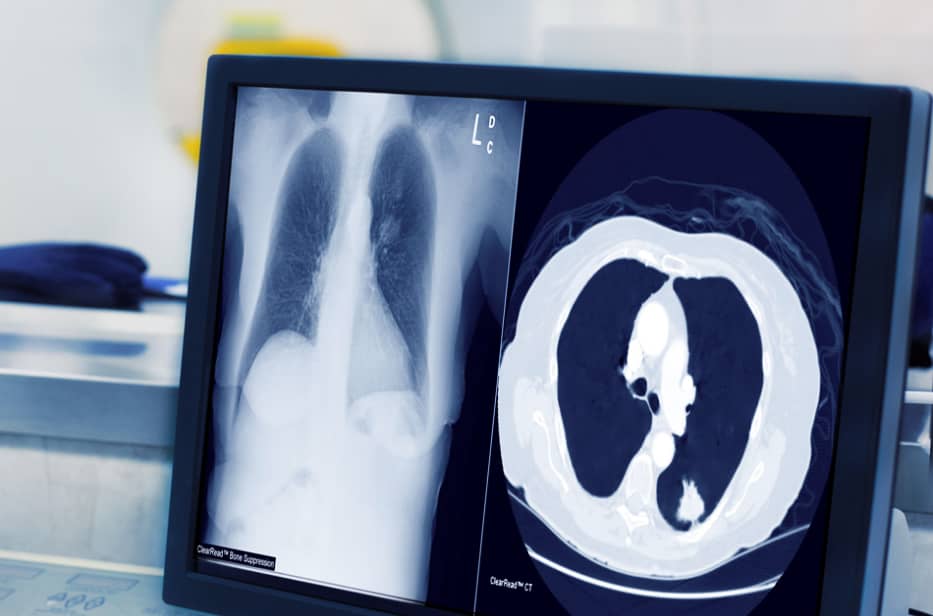
Riverain Technologies advanced AI imaging software is in use by leading healthcare organizations around the world.

Peer reviews, reference accounts, and independent studies confirm that ClearRead technology is positively impacting the field of radiology.
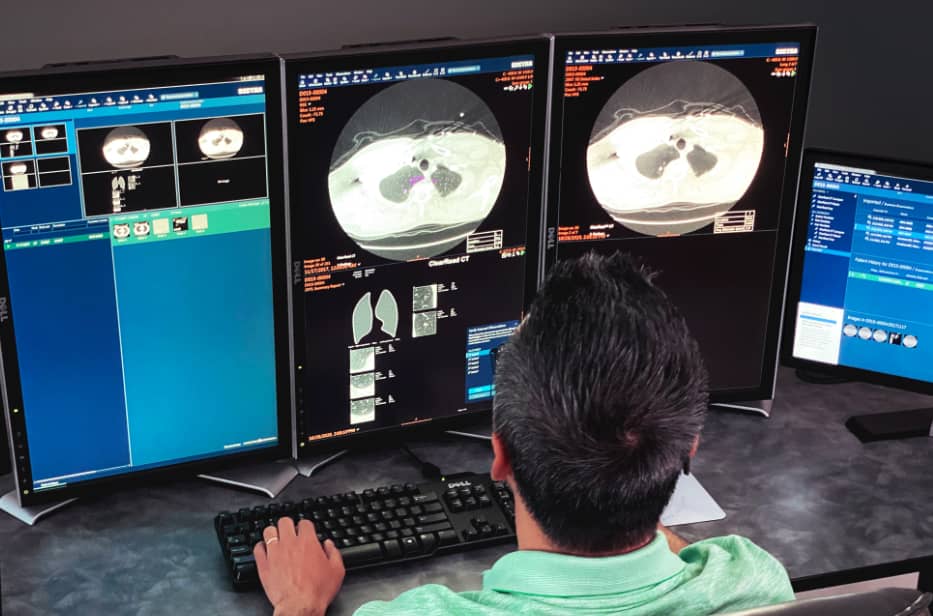
Managing your lung program can seem daunting, whether you’re getting going, growing, or managing workflow. We’ve curated resources for you.
Riverain’s ClearRead™ CT AI for pulmonary nodule detection and analysis to integrate Bialogics AIQ™ Performance Monitoring, to provide real-time AI performance, facilitating diagnostic and performance monitoring.

Aurora, Ontario and Dayton, OH – Nov 5, 2025 – Bialogics and Riverain Technologies today announced that the Bialogics AIQ Performance Monitoring framework will be integrated with the Riverain ClearRead CT cardiothoracic AI suite.
By integrating clinical findings with workflow and clinical utility data, the AIQ™ (AI Quality) Framework enables healthcare organizations and AI providers to adopt AI responsibly and transparently, anchoring decisions in a standardized framework of evidence-driven AI performance.
ClearRead™ CT is a cardiothoracic AI suite that reduces reading time and eases cognitive burden while improving the detection of missed nodules. A series of advanced AI algorithms is deployed and provide key insights and metrics on pulmonary nodules and coronary artery calcification.
This partnership represents a shared commitment to advancing AI transparency, performance, and clinical trust, enabling radiologists and health systems to understand better, measure, and improve the impact of AI in their daily practice.
“Integrating AI performance directly within the AI algorithms themselves establishes a new standard for transparency, accuracy, and accountability. By benchmarking every algorithm through a consistent and standardized methodology, we’re redefining how AI performance is understood, adopted, and optimized in lung screening and beyond”, says Jeff Vachon, President, Bialogics.
“The power of AI in diagnostic imaging grows when the patient journey is monitored and measured. Combining Bialogics AIQ framework with the ClearReadTM CT nodule detection and analysis tools brings new visibility into the performance of the tool and the patient journey of those with lung cancer and in the future, other lung health applications,” says Steve Worrell, CEO, Riverain Technologies.
Key Features of the Evidence-Driven AIQ Framework:
● Real-time Monitoring: Validates AI outputs against radiologist clinical findings for ongoing quality control and assurance.
● AIQ Score: A composite, weighted performance score reflecting clinical validity and operational efficiency, tailored to disease-specific applications. AIQ Scoring calculates the measurements of core performance indicators in real-time, including Concordance/ Discordance, Positive and Negative Concordance Rates (PCR/NCR), Positive and Negative Predictive Value (PPV/NPV), and Augmented Findings Rate (AFR), enabling evidence-based AI adoption.
● Workflow Optimization Metrics: Assesses AI’s impact on report turnaround time, productivity, and radiologist workload; translating technical performance into operational value and immediate assessment of ROI.
● Bias and Drift Detection: Identifies demographic disparities and predictive drift, ensuring AI performs reliably across populations and over time.
The ClearReadTM CT Cardiothoracic Suite:
ClearRead CT first suppresses vessels for an unimpaired view of the chest. Then, pulmonary nodules are detected, segmented, quantified, and compared with corresponding nodules on the prior exam. Finally, coronary artery calcification is quantified using the Agatston score. ClearRead CT findings, volumetrics, and scores are then included in the radiology report.
Demos will be available at RSNA booth #5628 (Bialogics) and #4711 (Riverain).
About Bialogics
Bialogics is a leading provider of data analytics and business intelligence solutions for the healthcare industry. With a mission is to revolutionize Medical Imaging by turning data into actionable insights that drive operational excellence, enhance productivity, and improve clinical and financial outcomes.
Contact:
Jeff Vachon, President
Bialogics Analytics
jvachon@bialogics.com
www.bialogics.com
About Riverain Technologies
Riverain Technologies is dedicated to transforming the field of radiology by addressing and eliminating delayed cardiothoracic diagnoses. As relentless innovators, Riverain empowers healthcare providers by streamlining diagnostic workflows, enhancing detection accuracy, and ultimately improving patient outcomes. With a steadfast commitment to advancing cardiothoracic care, Riverain Technologies is shaping the future of diagnostic excellence. For more information: https://www.riveraintech.com/
Contact:
Mandy Bayman, Director of Marketing
Riverain Technologies
mbayman@riveraintech.com
Our technology is revolutionizing radiology. We’d love to work with you to help create better outcomes for patients everywhere.
Subscribe to Riverain via Email
Stay up to date on the latest advancements
Riverain Technologies
3130 South Tech Blvd., Miamisburg, OH 45342
800-990-3387
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |