Our technology is revolutionizing radiology. We’d love to work with you to help create better outcomes for patients everywhere.
The first FDA-cleared device that supports concurrent reading, allowing for faster reading with proven, superior, automatic nodule detection.
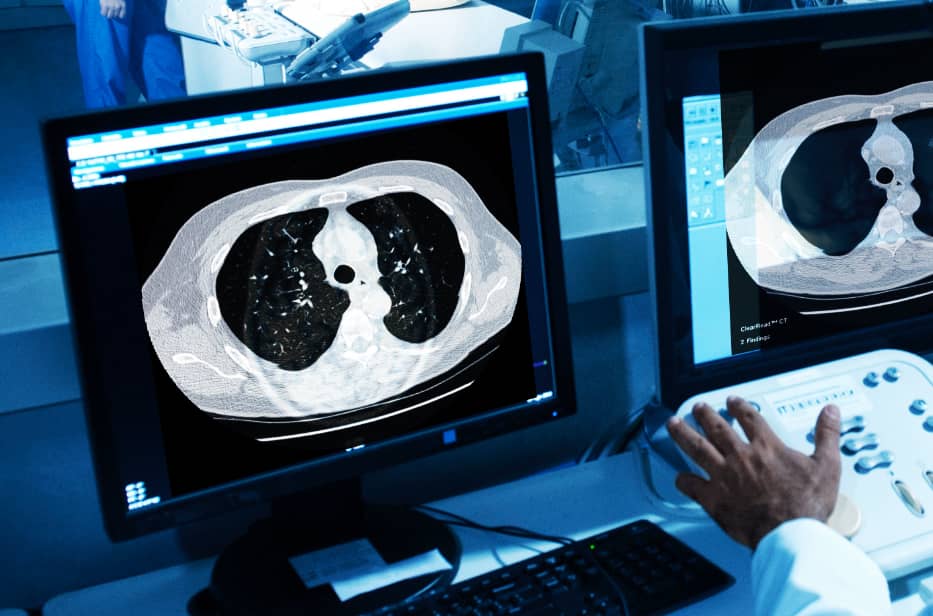
Our five FDA-cleared applications are designed to improve reading efficiency and accuracy across the hospital enterprise without additional equipment, procedures or radiation dose.
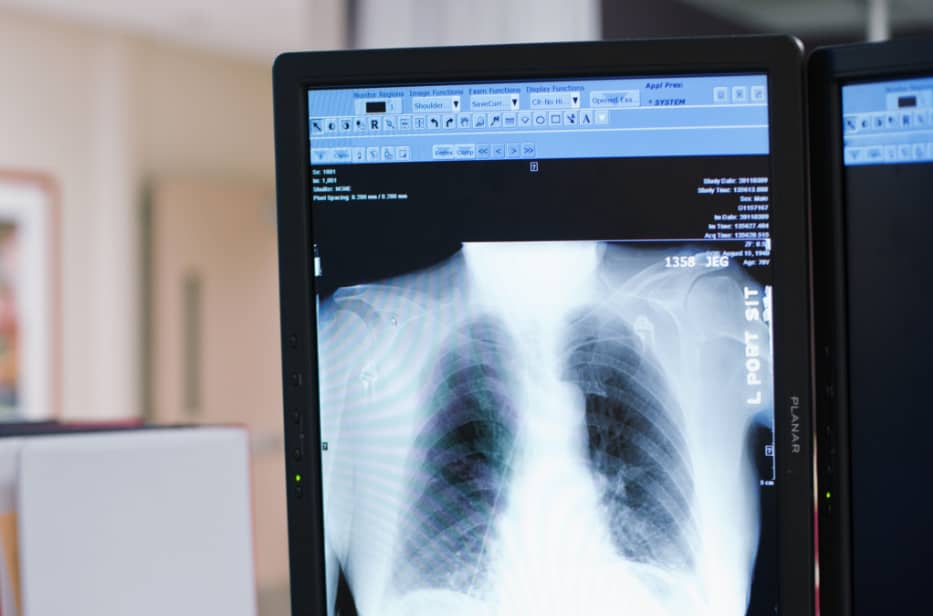
Riverain’s deep learning AI technology is FDA cleared, field tested, and clinically proven to provide real results in radiology.
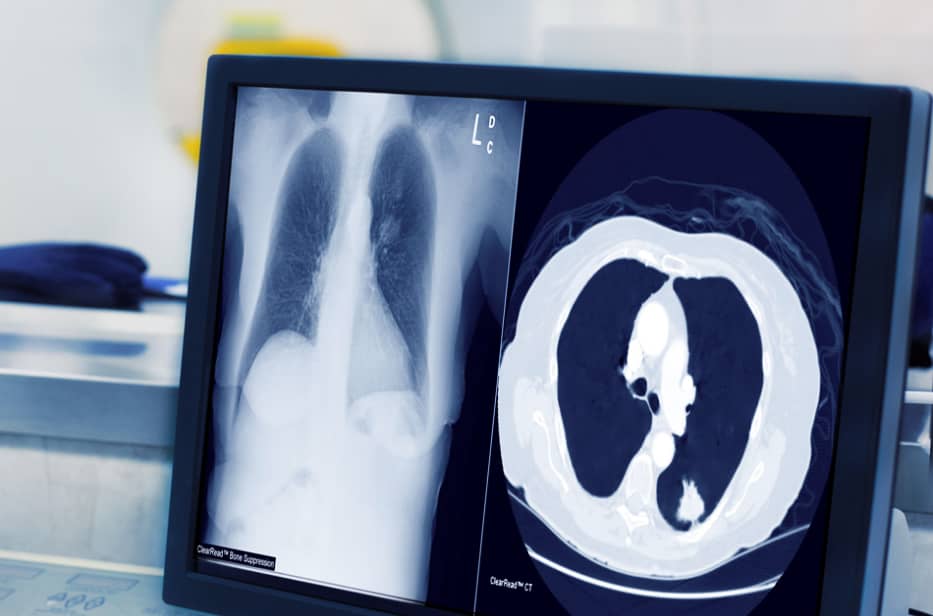
Riverain Technologies advanced AI imaging software is in use by leading healthcare organizations around the world.

Peer reviews, reference accounts, and independent studies confirm that ClearRead technology is positively impacting the field of radiology.
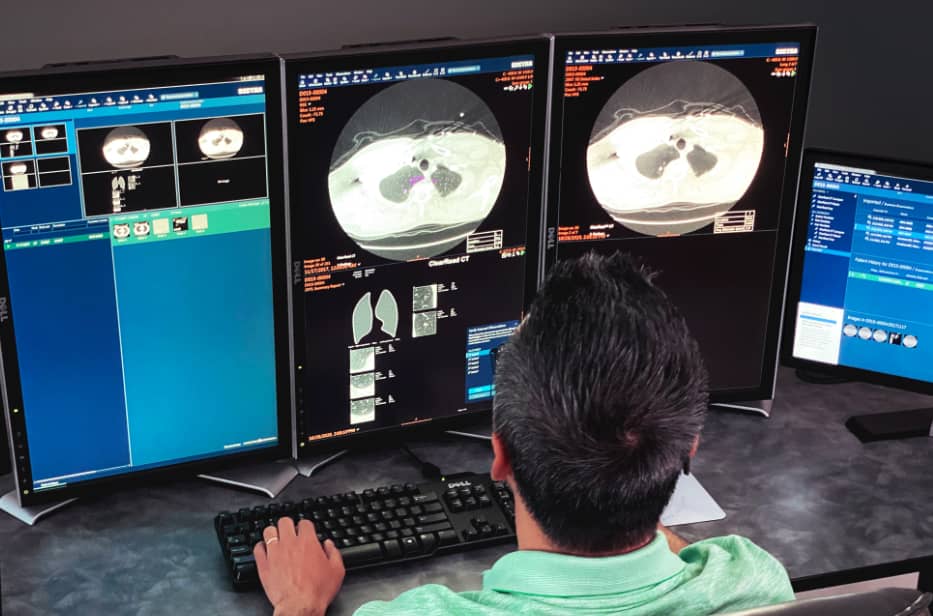
Managing your lung program can seem daunting, whether you’re getting going, growing, or managing workflow. We’ve curated resources for you.
Riverain’s cardiothoracic AI suite is now available to sponsors with VIDA’s clinical trial imaging services, through the VIDA Intelligence PlatformTM.

Chicago, IL, Nov 30, 2025 – VIDA Diagnostics, Inc. (VIDA), the leader in clinical imaging intelligence, today announced a strategic partnership with Riverain Technologies at this year’s Radiologic Society of North America (RSNA) meeting. This collaboration integrates Riverain’s ClearRead™ CT cardiothoracic AI imaging analysis suite through the VIDA Intelligence Platform, a modern trial imaging management solution designed to ensure high-quality biomarker data and operational excellence on a global scale.
Riverain joins an exceptional ecosystem of AI partners, following successful integrations with Thirona, National Jewish Health, and Polarean. By unifying the workflows accessing these best-in-class technologies through the VIDA Intelligence Platform, VIDA provides pharmaceutical science teams a complete arsenal of precision imaging biomarker services. This centralized approach eases specialized AI vendor management and standardizes biomarker outputs, leveraging a proven secure infrastructure used globally by thousands of participating trial sites.
“Pharmaceutical sponsors are seeking a deeper understanding of their therapies, patients, and the complex interplay among major diseases like COPD, cardiovascular disease, and lung cancer,” said Susan Wood, PhD, President and CEO of VIDA. “Our partnership with Riverain delivers the type of deeply clinical imaging tools necessary to drive next generation therapies.”
“We are excited to bring our renowned ClearRead AI technology to VIDA’s platform-driven clinical trial solution, extending our solution to the rapidly growing life sciences market,” said Steve Worrell, CEO of Riverain Technologies. “By combining our strengths, we can empower pharmaceutical vendors with stronger data to accelerate therapeutic breakthroughs for patients worldwide.”
This partnership complements VIDA’s precision imaging portfolio in addressing the “Big Three” diseases of the chest—Cardiovascular (CVD), Respiratory/COPD, and Lung Cancer—which collectively account for 40% of global deaths annually1. As pharmaceutical demand shifts toward a more comprehensive patient evaluation, the ability to analyze comorbidities is essential. With data showing that a COPD diagnosis doubles heart attack risk² and that 70-80% of lung cancer cases are preceded by COPD³, VIDA’s platform now provides the multi-disease visibility that modern medicine—and the market—demands.
The ClearRead CT technology deploys a series of advanced AI algorithms that provide key insights and metrics on pulmonary nodules, coronary artery calcification (CAC), and hypodense lung tissue*. The quantification of these biomarkers is a valueable tool in the detection and treatment of the big three diseases of the chest.
About VIDA
VIDA is a clinical imaging intelligence company, accelerating the approval and adoption of life-saving therapies to patients through its AI-powered digital biomarker solution. With best-of-breed imaging biomarkers and a modern approach to trial imaging management, the VIDA Intelligence Platform is helping biopharma sponsors leverage the power of quantitative imaging at scale. VIDA’s platform technology powers OSIC Cloud, the world’s largest ILD imaging data repository. Learn more at https://vidalung.ai. Follow @vidalung on X and LinkedIn.
About Riverain
Riverain Technologies is dedicated to transforming the field of radiology by addressing and eliminating delayed cardiothoracic diagnoses. As relentless innovators, Riverain empowers healthcare providers by streamlining diagnostic workflows, enhancing detection accuracy, and ultimately improving patient outcomes. With a steadfast commitment to advancing cardiothoracic care, Riverain Technologies is shaping the future of diagnostic excellence. For more information: https://www.riveraintech.com/
Sources
1 – World Health Organization: CVD (~32%), COPD (~5%), lung cancer (~3%)
2 – Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Therapeutic Advances in Respiratory Disease. 2018;12:1753465817750524-1753465817750524.
3 – Parris BA, O’Farrell HE, Fong KM, Yang IA. Chronic obstructive pulmonary disease (COPD) and lung cancer: common pathways for pathogenesis. Journal of Thoracic Disease. 2019;11(S17):S2155-S2172.
*ClearRead CT LTA for hypodense lung tissue is pending FDA clearance.
Our technology is revolutionizing radiology. We’d love to work with you to help create better outcomes for patients everywhere.
Subscribe to Riverain via Email
Stay up to date on the latest advancements
Riverain Technologies
3130 South Tech Blvd., Miamisburg, OH 45342
800-990-3387
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |